Navigation auf uzh.ch
Navigation auf uzh.ch
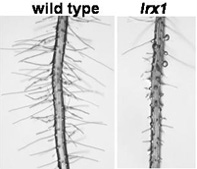
The plant cell wall is a complex supramolecular structure formed by polysaccharides and proteins. The aim of our work is to characterize genes / proteins that are involved in cell wall formation. LRX proteins have a regulatory function during this process. These extracellular proteins consist of a Leucine-Rich-Repeat (LRR) and an extensin domain (1). LRR-domains are frequently found in proteins with signaling or regulatory functions. The extensin domain is a structural cell-wall protein domain, presumably important for anchoring of the protein in the cell wall matrix. LRX1 and LRX2 of Arabidopsis thaliana are expressed in root hairs and mutations in the genes lead to aberrant cell wall formation and hence deformed root hair structures (2,3).
The aim of this project is the functional analysis of LRX1. In one approach, we attempt to identify the interaction partner of LRX1. In a second approach, we are characterizing the extensin domain of LRX1. This highly repetitive domain is crucial for LRX1 function and leads to insolubilization of the protein in the cell wall, most likely via oxidative cross-linking of tyrosine residues. The aim is to identify the domain(s) and eventually individual amino acid residue(s) in the extensin moiety that are crucial for the proper function of LRX1.
(1) Baumberger N, Doesseger B, Guyot R, Diet A, Parsons RL, Clark MA,
Simmons MP, Bedinger P, Goff SA, Ringli C, Keller B (2003) Whole-genome
comparison of leucine-rich repeat extensins in Arabidopsis and rice: a
conserved family of cell wall proteins form a vegetative and a
reproductive clade. Plant Physiol 131: 1313-1326
(2) Baumberger N, Ringli C, and Keller B (2001) The chimeric
leucine-rich repeat/extensin cell wall protein LRX1 is required for root
hair morphogenesis in Arabidopsis thaliana. Genes & Dev 15:
1128-1139
(3) Baumberger N, Steiner M, Ryser U, Keller B, Ringli C (2003)
Synergistic interaction of the two paralogous Arabidopsis genes LRX1
and LRX2 in cell wall formation during root hair development.
Plant J 35: 71-81
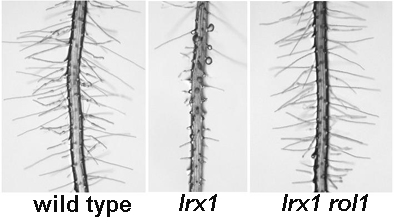
Diet A, Link B, Seifert GJ, Schellenberger B, Pauly M, Reiter W-D, Ringli C (2006) The root hair cell wall formation mutant lrx1 is suppressed by mutations in the RHM1 gene encoding a UDP-L- rhamnose synthase. Plant Cell 18: 1630-1641

The mechanical properties of the cell wall regulates the growth rate and the final shape of an individual cell. Even though the composition of different cell walls is quite well understood, there is limited information available concerning the contribution of the different polysaccharidic and proteinaceous components of the wall to its properties. In this project, we are aiming at modifying the composition of cell walls and quantify the effects of these changes on the physical and growth properties of the cell.