Projects
Canonical Functions of Polycomb Group Proteins
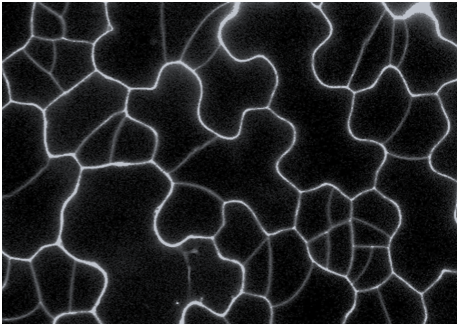
In multicellular organisms, proper coordination of cell growth and differentiation is essential from embryogenesis through to adulthood. Polycomb group (PcG) proteins—particularly those forming the Polycomb Repressive Complex 2 (PRC2)—are key developmental regulators. These proteins are conserved across kingdoms, from plants to Drosophila, mice, and humans, and they function through epigenetic mechanisms to modulate transcription. The canonical role of PRC2 involves the deposition of the histone mark H3K27me3 (trimethylation of lysine 27 on histone H3), which leads to transcriptional repression.
Our lab investigates the canonical functions of PRC2 using a combination of genetic, epigenetic, genomic, and molecular approaches. Our research aims to identify PRC2 recruitment factors and uncover their mechanisms of action.
We focus primarily on seed development and embryogenesis, with particular interest in the spatial and temporal regulation of cell identity, cell cycle progression, and tissue and organ patterning. To work with these small and challenging tissues, we have optimized methods to profile H3K27me3 and other histone modifications from very limited amounts of plant material.
|
|
|
Images by Yixuan Fu
Non-Canonical Functions of Polycomb Group Proteins
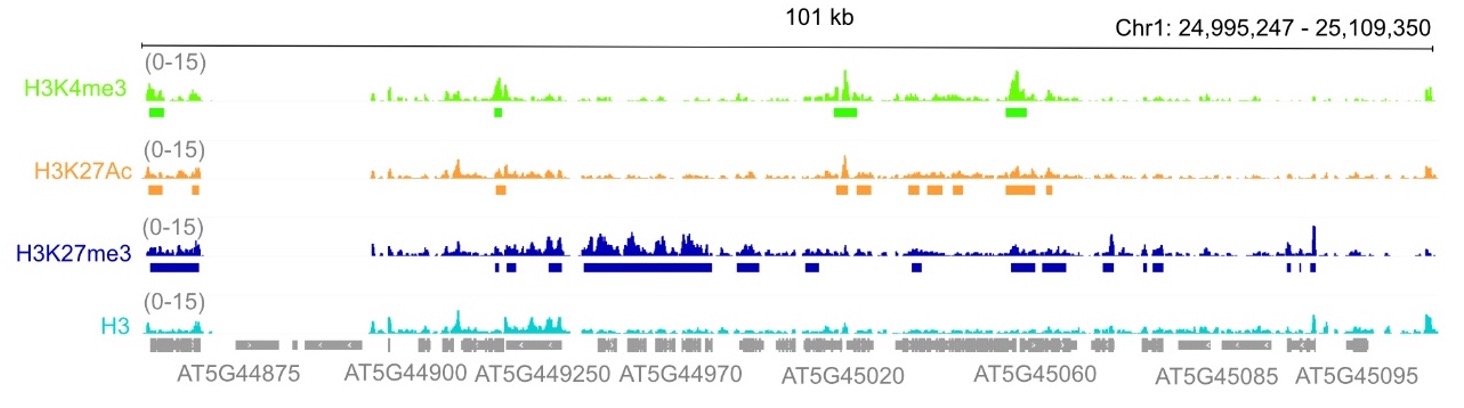
Despite extensive research, how the Polycomb Repressive Complex 2 (PRC2) achieves precise, cell-type–specific, and developmentally tailored transcriptional regulation remains largely unresolved, especially in plants. The most commonly used approach to study PRC2 activity is genome-wide profiling of H3K27me3, the histone modification deposited by PRC2. While the presence or absence of this mark is often used as a proxy for PRC2 function, this strategy captures only its canonical chromatin-based activity and overlooks non-canonical roles—particularly the potential methylation of non-histone targets.
Indeed, in mammals, the PRC2 catalytic subunit EZH2 has been shown to methylate several non-histone proteins both within and beyond the PRC2 complex, in both nuclear and cytoplasmic compartments. These modifications regulate a variety of cellular processes, including protein stability, interaction affinity, and cell proliferation.
In this project, we aim to explore these under-investigated aspects of PRC2 biology by identifying non-histone targets that may contribute to its regulatory roles during plant development. Using a combination of molecular and proteomic approaches, we compare plants expressing wild-type PRC2, catalytically inactive (enzyme-dead) PRC2, and hyperactive PRC2 variants to uncover novel PRC2 targets and mechanisms of action.
Image by Janik Scotton
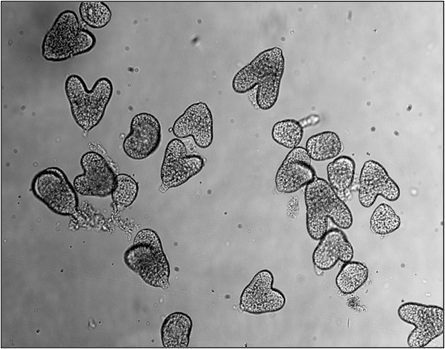
Acquisition of cell fate during embryogenesis
The unicellular zygote is totipotent—capable of giving rise to all cell types of the organism. As development proceeds, the zygote undergoes successive divisions and the embryo increases in size. Cell types begin to be specified in a highly regulated spatial and temporal manner. In plants, where cells are immobile due to rigid cell walls, the precise coordination of when and where cell fate is determined is especially critical.
In this project, we investigate the temporal hierarchy of histone modification dynamics—both deposition and removal—throughout embryogenesis. We focus on a well-characterized and visually accessible developmental pathway: stomatal development. Stomatal precursor cells emerge on the surface of the embryonic epidermis at a defined developmental stage, making them an ideal system for studying early cell fate decisions.
By manipulating the spatial and temporal timing of stomatal precursor differentiation during embryogenesis, we aim to uncover the epigenetic mechanisms that govern the acquisition of new cell identities.
- Image from Max Schwarze
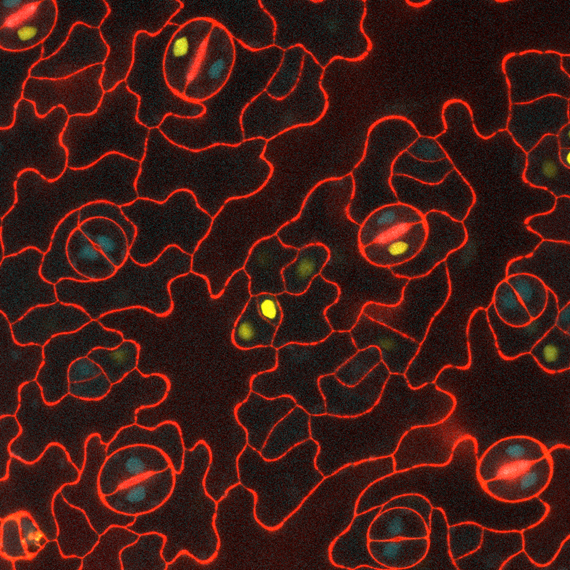
Acquisition of a new cell fate during asymmetric cell division
Multicellular organisms, including both animals and plants, rely on populations of stem cells to generate diverse cell types that form tissues and organs with specialized functions. Although these cells share identical genetic information, they can initiate distinct developmental programs. Understanding how this cellular diversity arises remains one of the most fundamental unresolved questions in developmental biology.
A defining feature of stem cells is their ability to divide asymmetrically. Unlike typical proliferative divisions in differentiated cells, asymmetric cell division (ACD) produces two daughter cells with different developmental potentials, sizes, and identities. One daughter retains the stem cell fate and self-renewal capacity of the mother cell, while the other embarks on a path toward differentiation. This evolutionarily conserved mechanism operates across life forms—from the budding of Saccharomyces cerevisiae, to zygotic division in animals and plants, to the formation of the nervous system in mammals.
Despite the importance of ACD in generating cellular diversity, the mechanisms that trigger an asymmetric rather than symmetric division are still poorly understood. This project aims to investigate the molecular and cellular factors responsible for breaking symmetry during ACD. In particular, it focuses on identifying the signals and determinants that enable one daughter cell to retain stem cell identity while directing the other toward differentiation.
We use the asymmetric division that produce the stomatal lineage as model system.
Images by Sara Simonini
Epigenetic basis of flowering time in grasses
Climate change increasingly threatens global food production, affecting plant growth, development, and reproductive success. One of the most climate-sensitive developmental transitions in plants is the timing of flowering. Many key crops—including cereals—require a prolonged period of cold to become competent to flower in spring. This process, known as vernalization or the "memory of winter," enables plants to align flowering with seasonal cues, ensuring reproductive success.
However, climate-induced fluctuations in winter duration and temperature pose serious challenges for crops that depend on vernalization. The key question is whether crops can adjust their vernalization response fast enough to keep pace with increasingly unpredictable winters.
In these projects, we aim to develop strategies to modulate the vernalization requirement by temperatire and light in members of the Poaceae family, thereby improving yield resilience under variable winter conditions. We use barley, perennial ryegrass and wheat as models, and we work in close collaboration with Syngenta. Our main focus is the of the epigenetic state of vernalization genes during embryogenesis, and the resposiveness of flowering genes to photoperiod. Our goals is to identify commercially available compounds that can be applied either in the field or in tissue culture to fine-tune vernalization responses in crops.
Images by Eugenio Reale
Thermotolerance in cereals
Climate change poses a serious threat to food production, with both short- and long-term impacts on plant growth, development, and reproductive success. Among the most acute manifestations of climate change are heat waves, which are becoming increasingly frequent and intense. Many crop species, including key cereals, require a specific temperature range for proper development. Exposure to elevated temperatures can severely compromise seed formation, and heat stress has already led to major yield losses worldwide.
To cope with heat stress, plants activate a protective response known as thermotolerance - the ability to endure and recover from high-temperature exposure. This response involves the induction of specific heat-protective proteins, whose expression is tightly regulated at the epigenetic level. High temperatures can trigger heritable epigenetic changes that enable faster and stronger activation of thermotolerance genes in future stress events.
In this project, we use barley as model system and we aim to identify the key thermotolerance genes that protect developing embryos and to characterize the epigenetic mechanisms that regulate their expression. Our goal is to epigenetically prime these genes to ensure their strong and constitutive expression throughout embryogenesis. By embedding this molecular resilience early in development, we seek to create plants that are better equipped to withstand heat stress and ensure robust seed development under rising global temperatures.
Image from Sara Simonini
Regulation of the cell division cycle during plant reproduction
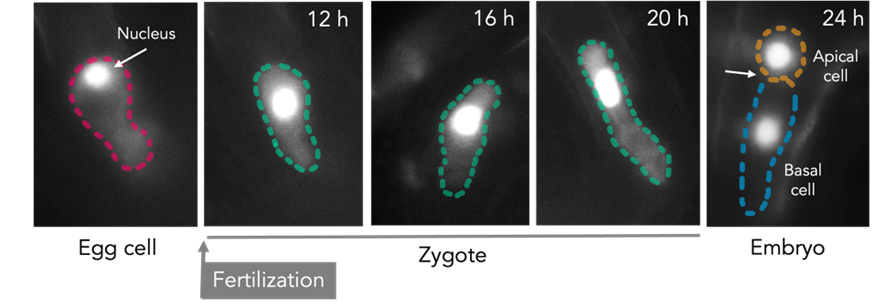
All multicellular life begins with the union of two gametes, yet how a single fertilized cell—the zygote—transitions from dormancy to a fully patterned embryo remains one of the great unanswered questions in developmental biology. In plants, this transition is especially striking: the zygote not only re-enters the cell cycle but also establishes polarity and undergoes an asymmetric division that sets the foundation for all future development. Despite its central role in plant reproduction, the molecular mechanisms governing these early steps—cell cycle reactivation, zygote polarization, and apical–basal fate specification—are poorly understood.
In this project we aim to dissect these fundamental processes in the model plant Arabidopsis thaliana, using a combination of techniques including laser-assisted microdissection followed by transcriptomic and proteomics of rare cells such as egg cells and zygotes, high-resolution light-sheet microscopy for live imaging of zygote development, and ultra-sensitive transcriptomic, proteomic, and epigenomic profiling of rare cell populations.
Our goal is to uncover the molecular and epigenetic logic of early embryogenesis, and to use this knowledge understand how to bypass fertilization and engineer asexual seed formation.
Images by Sara Simonini
Open positions
Bachelor and master thesis projects are available in the lab.
Topics include epigenetic regulation of gene expression in the contest of seed and embryo development, Polycomb proteins mode of actions, epigenetic-based memory mechanisms, and transcriptional regulation of plant development in general.
The students will become familiar with cutting-edge methods in molecular biology, genetics, developmental biology, and proteomics. Projects will be also tailored to follow inclinations and preferences from the student.
If interested, please send an email to sara.simonini@botinst.uzh.ch or pass by the lab (BOT P2.14).